旋转电极技术简介
电化学中的强制对流
电化学实验通常需要采用强制对流来加强传质并确保反应物分布均匀。采用 旋转盘电极 旋转盘电极可引入流体动力学条件,对实验结果产生重大影响。电极的旋转会产生受控的电解质流,促进反应物向电极表面移动,而生成物则远离电极表面。这种动态环境对于在各种电化学研究中保持稳态条件和获得可重复的结果至关重要。
在强制对流中使用旋转电极尤其适用于传统静态解决方案无法提供充分传质的情况。通过创造层流条件,这些电极可确保反应物在电极表面均匀分布,从而最大限度地减少浓度梯度,提高电化学反应的效率。这种方法在电催化等应用中尤其有用,因为在这些应用中,反应速率高度依赖于电极表面的反应物供应情况。
此外,通过控制电极的旋转速度,研究人员还可以操纵流体动力学条件,从而模拟从层流到湍流的各种流动状态。这种多功能性使旋转电极成为研究不同流动条件下电化学过程不可或缺的工具,为研究动态环境中反应物和产物的行为提供了宝贵的见解。
旋转电极的应用
了解静态和动态条件
要完全掌握旋转电极在电化学中的应用,关键是要区分静态溶液条件和流体动力学条件。此外,了解层流和湍流的区别对于准确模拟和分析各种电化学过程也至关重要。
在静态溶液条件下,反应物主要通过扩散、迁移和自然对流进行移动。缺乏强制对流会导致反应物分布不均匀和传质效率降低,从而直接影响电流测量的准确性和电化学实验的整体结果。
另一方面,通过使用旋转电极实现的流体动力学条件则引入了强制对流。这使得反应物的分布更加可控和均匀,从而提高了传质效率。旋转电极系统可产生层流或湍流,各有不同的特点和应用。
-
层流:在层流条件下,流体在平行层中运动,确保了平滑和可预测的流动模式。这种均匀性特别有利于获得稳态最大电流,因此非常适合基础电化学实验和电催化研究。
-
湍流:湍流的特点是混乱和不规则的流体运动,通常在旋转电极的边缘产生。这种情况适用于模拟复杂的流动环境,例如在管道研究等工业应用中,不可预测的流动模式是常态。
通过区分这些条件,研究人员可以选择适当类型的旋转电极和流动条件,以最好地满足其特定的实验需求,从而优化电化学研究的结果。
旋转电极的类型
在电化学领域,旋转电极在创造受控流体动力学条件方面发挥着关键作用,而受控流体动力学条件对于各种实验装置来说至关重要。旋转电极的三种主要类型是:旋转圆盘电极(RDE)、 旋转环盘电极 (RRDE) 和旋转圆柱电极 (RCE)。每种类型的电极都具有不同的用途和应用场景,为电化学研究领域做出了独特的贡献。
旋转圆盘电极 (RDE)
RDE 是电化学实验,尤其是流体动力伏安法实验的基础。这些电极由嵌入惰性非导电材料中的导电圆盘组成,然后连接到电机上以精确控制旋转速度。旋转会促使分析物流到电极上,从而促进对氧化还原化学反应机制的研究。RDE 用途广泛,可用于基础实验、电催化研究和传感器开发。

旋转环盘电极(RRDE)
RRDE 更为复杂,同时具有圆盘电极和环形电极。虽然圆盘的功能与 RDE 类似,但环形电极可以进行额外的测量,这使得 RRDE 对于电催化实验和电化学反应机制的研究至关重要。在实验过程中,圆环可以处于非激活状态,从而有效地将 RRDE 转变为 RDE,为实验设计提供了灵活性。
旋转圆柱电极 (RCE)
RCE 主要用于腐蚀研究和工业环境中的流动条件建模。这些电极可以模拟包括湍流在内的复杂流动模式,尤其适用于管道研究等工业应用。圆柱形电极确保了反应物的均匀分布和有效的物质转移,使 RCE 成为了解电化学动态条件不可或缺的工具。
每种旋转电极都具有独特的优势,可满足电化学研究和工业应用的不同需求。通过了解它们的特定作用和能力,研究人员可以更好地设计实验,探索和优化各种流动条件下的电化学过程。
电化学中的静态解决方案
传质机制
在静态溶液中,推动传质的主要机制包括扩散、迁移和自然对流。这些过程共同影响着电化学实验中反应物的移动和电流测量的准确性。
-
扩散 是指在浓度梯度的驱动下,颗粒从浓度较高的区域向浓度较低的区域移动。这种机制在静态溶液中至关重要,因为在静态溶液中,由于缺乏外部搅拌,反应物必须自发地移动到电极表面。
-
迁移 发生迁移的原因是工作电极和参比电极之间的电位差产生的电场。溶液中的离子被该电场吸引或排斥,从而促进了整体的质量转移。
-
自然对流 是指由温度梯度或浓度差引起的密度差导致的流体运动。在静态溶液中,自然对流可能是一个重要因素,尤其是在体积较大或温度较高的情况下。
这些机制的相互作用会影响传质的速度和效率,从而影响电化学反应以及电流和电位等测量的准确性。了解这些机制对于优化实验条件和准确解释结果至关重要。
峰值电流和扫描速率
在静态溶液中,峰值电流明显受到伏安实验扫描速率的影响。随着扫描速率的增加,峰值电流也会增加,Fe²⁺ 氧化的伏安图生动地证明了这一现象。这种关系可归因于几个因素,主要是传质增强和电极表面的反应物增多。
当扫描速率加快时,电极在每个电位上花费的时间减少,导致电荷积累更快。由于电极能够在相同的时间内将更多的反应物从溶液中吸附到表面,因此这种快速积累会产生更高的峰值电流。扫描速率越高,扩散层越薄,从而促进了这一过程,确保更有效地将 Fe²⁺ 离子转移到电极上。
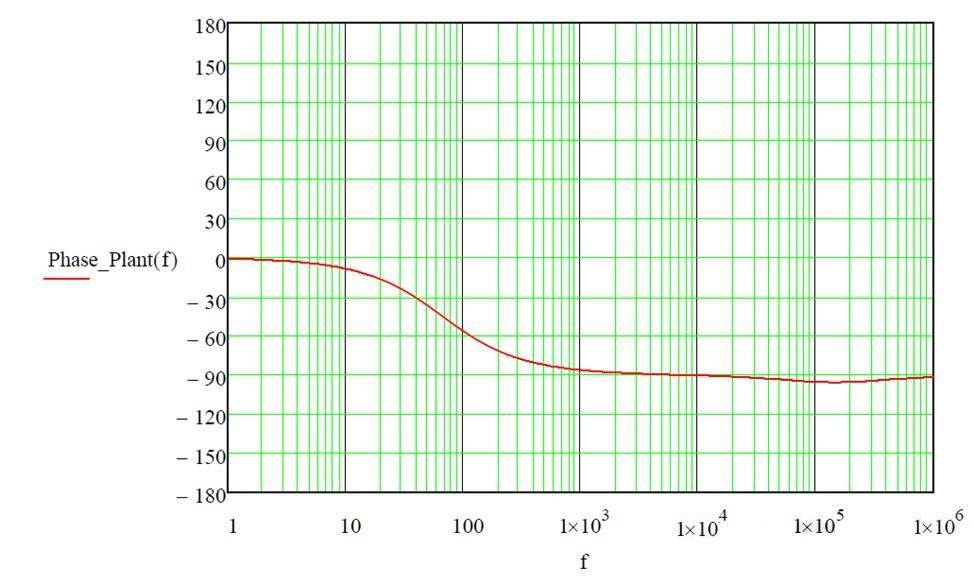
此外,电化学反应的动力学限制也起着至关重要的作用。在较快的扫描速率下,反应动力学通常会达到极限,从而导致观测到更大的电流。这在反应受扩散限制的系统中尤为明显,因为扫描速率的增加会加剧这种限制,导致观测到的电流成比例增加。
总之,静态溶液中的峰值电流是扫描速率的直接函数,由于改善了传质和动力学限制,扫描速率越快,电流越大。这种关系是理解静态条件下电化学系统行为的基础,也是设计伏安法实验的关键考虑因素。
电化学中的动态条件
层流
层流是电化学中一个关键的流体动力学条件,通过使用旋转电极可对其进行精细控制。这种方法有助于在电极表面形成反应物的均匀分布,这种现象对于实现高效的物质转移至关重要。反应物分布的均匀性不仅仅是副产品,而是精确控制流动动力学的有意结果。
在电化学实验中,旋转电极系统可确保反应物持续、可预测地输送到电极上,从而最大限度地减少波动,提高实验结果的可重复性。在稳态电流至关重要的研究中,这种可预测性尤其有利,因为它可以准确测量和解释电化学反应。
这种效率背后的机制在于层流本身的性质。湍流的特点是混乱和不可预测的运动,而层流则不同,它表现出平滑、有序的流体层,不会混合。这种有序运动可确保反应物以受控方式被输送到电极,从而产生稳态最大电流。这种稳态条件对许多电化学应用(包括电催化和传感器开发)至关重要,因为在这些应用中,稳定可靠的数据对准确分析和解释至关重要。
此外,旋转电极在创造层流条件方面的作用不仅限于反应物的分布。它还会影响整体传质速率,而传质速率是决定电化学反应速率的关键参数。通过保持层流,研究人员可以更好地控制和优化传质过程,从而提高电化学系统的整体效率。
总之,通过旋转电极实现的层流不仅能确保反应物的均匀分布,还能促进有效的物质转移,从而产生稳态最大电流。从基础实验到电催化和传感器开发中的高级应用,这种可控和可预测的流动条件对于各种电化学研究都是不可或缺的。
湍流
湍流是旋转电极两侧产生的一种现象,它带来了错综复杂的流动模式,对于复制现实世界中的工业场景(如管道研究)非常有价值。这种复杂性使研究人员能够模拟和了解流体在动态条件下的行为,这对于优化工业流程至关重要。
在电化学方面,湍流可增强反应物的混合,从而更准确地测量传质速率和反应动力学。层流可保持稳定且可预测的模式,而湍流则不同,它引入了随机的漩涡运动,可显著影响电极表面附近化学物质的扩散和对流。
例如,在研究管道腐蚀时,模拟湍流条件可以深入了解不同材料和涂层在压力下的表现,从而有助于开发更耐用、更高效的保护措施。这种能力还可扩展到其他工业应用,如反应器中的流体动力学和化学合成中催化剂的性能评估。
通过旋转电极产生湍流不仅仅是一项理论工作,它还是一种实用工具,在实验室实验和工业现实之间架起了一座桥梁。通过创造这些复杂的流动条件,研究人员可以更好地预测和缓解实际操作中面临的挑战,最终为建立更强大、更可靠的工业系统做出贡献。
旋转电极的具体应用
旋转盘电极 (RDE)
从基础实验到先进的电催化研究和传感器开发,旋转盘电极 (RDE) 在众多电化学应用中发挥着关键作用。这些电极在三电极系统中发挥作用,在实验过程中,圆盘电极的旋转可确保分析物稳定地流向电极表面。这种受控的质量传输对于催化剂评估和燃料电池研究等需要精确流体力学条件的实验至关重要。
在电化学领域,RDE 是研究氧化还原化学和其他化学现象相关反应机制不可或缺的工具。旋转盘电极能够保持反应物稳定地流向电极表面,从而获得更准确、更可重复的结果。这在腐蚀研究等应用中尤为重要,因为反应物的均匀分布可确保全面了解电化学过程。
RDE 的结构包括一个嵌入惰性非导电聚合物或树脂中的导电圆盘,该圆盘与一个能够精细控制旋转速率的电机相连。圆盘的材料可以多种多样,通常由贵金属或玻璃碳制成,但也可以根据具体的实验要求使用其他导电材料。材料选择的多样性进一步提高了 RDE 在各种电化学研究中的适用性。
总之,RDE 在推动我们了解受控流体力学条件下的电化学反应方面发挥着至关重要的作用,使其成为学术研究和工业应用的重要工具。
旋转环盘电极 (RRDE)
旋转环盘电极 (RRDE) 是电催化和电化学反应机理研究领域不可或缺的工具。其独特的设计结合了中央圆盘电极和外环电极,可同时检测电子转移反应的反应物和产物。这种双重检测能力在阐明复杂的反应途径和识别传统技术可能无法观察到的中间产物方面尤为有利。
RRDE 的主要应用之一是评估氧还原反应 (ORR)、氮还原反应和二氧化碳还原等关键过程的电催化剂活性。环形电极还可用作 pH 传感器,让人们深入了解圆盘上发生的反应所导致的 pH 变化。这种多功能性使 RRDE 成为学术研究和工业应用中的多用途仪器。
尽管 RRDE 做出了重大贡献,但其商业应用仅限于少数几种电极材料,如玻璃碳、铂和金。这种局限性凸显了进一步研究和开发的必要性,以扩大适合各种实验要求的材料和配置的范围。
总之,RRDE 为研究电化学反应提供了一种复杂的方法,可提供有价值的机理见解,并能对一系列关键过程的电催化剂性能进行评估。
旋转圆柱电极 (RCE)
旋转圆柱电极 (RCE) 是一种特殊的旋转电极,在腐蚀研究和工业环境中的流动条件建模方面发挥着关键作用。与旋转圆盘电极 (RDE) 和旋转环形圆盘电极 (RRDE) 等其他旋转电极不同,RCE 设计用于模拟更复杂的流体动力学场景,尤其是涉及湍流的场景。这种能力对于模拟管道和化学处理装置等工业环境中经常遇到的恶劣多变条件至关重要。
在腐蚀研究中,RCE 对于研究流体动力学对材料腐蚀速率的影响非常重要。通过以不同的速度旋转,RCE 可以产生层流或湍流条件,使研究人员能够观察不同的流态如何影响腐蚀过程。这对于制定减缓腐蚀的策略至关重要,因为在材料暴露于侵蚀性流体和不同流速的行业中,腐蚀是一个重大问题。
此外,RCE 不仅限于腐蚀研究,还可用于模拟真实世界工业场景的流动条件建模。例如,它们可用于模拟腐蚀性流体在管道中的流动,为了解不同流速和流体特性如何影响材料降解提供宝贵的信息。这些信息对于从事工业基础设施设计和维护工作的工程师和科学家来说非常宝贵,可确保材料能够经受住连续运行的严酷考验。
总之,RCE 是电化学领域不可或缺的工具,具有研究和模拟复杂流动条件的独特能力,可直接用于工业腐蚀研究和流体动力学模拟。
总结和结论
旋转电极技术的重要性
旋转电极技术是电化学研究的重要工具,具有模拟和研究层流和湍流条件的独特能力。这种多功能性是各种电化学研究不可或缺的,因为了解和控制流体动力学会对实验结果产生重大影响。
在层流条件下,旋转电极可确保反应物在电极表面均匀分布,从而促进有效的传质,实现稳态最大电流。这种均匀性对于精确测量和准确解释电化学数据至关重要,尤其是在电催化和传感器开发等应用中。
另一方面,旋转电极产生的湍流条件为流动模式带来了复杂性和可变性,模拟了真实世界的工业环境。在涉及管道流动、腐蚀研究和其他工业应用的研究中,这种能力尤为重要,因为在这些研究中,不可预测的流动动态是常态而非例外。
在这两种流动状态之间切换的能力使研究人员能够弥合受控实验室环境与不可预测的工业流程之间的差距。通过这种方式,旋转电极可以全面了解不同流体动力学条件下的电化学现象,从而提高电化学研究的预测能力和实际应用性。
总之,旋转电极具有模拟层流和湍流条件的双重能力,是现代电化学研究的重要工具,弥补了理论模型和实际应用之间的差距。
相关产品
- 旋转铂圆盘电极,用于电化学应用
- RRDE 旋转圆盘(圆环圆盘)电极 / 兼容 PINE、日本 ALS、瑞士 Metrohm 玻碳铂
- 金属圆盘电极 电化学电极
- 石墨圆盘棒片电极 电化学石墨电极
- 实验室用甘汞银氯化汞硫酸盐参比电极




















