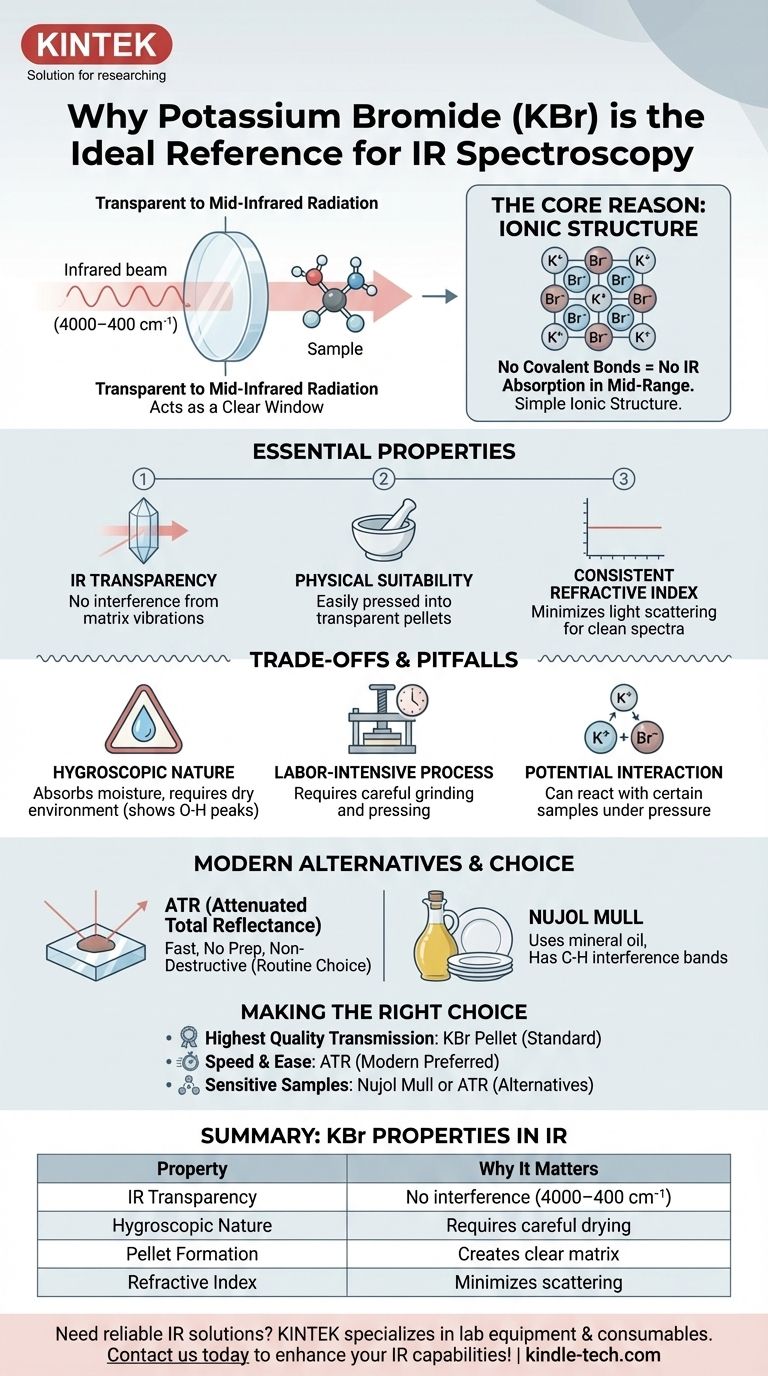
简而言之,溴化钾(KBr)在红外光谱中用作参比化合物和样品基质,因为它对红外辐射是透明的。其简单的离子结构没有在典型的中红外范围(4000–400 cm⁻¹)内吸收能量的分子振动。这种独特的特性使其能够充当一个清晰的“窗口”,容纳目标样品,以便光谱仪能够测量样品的谱图而不会受到干扰。
使用KBr的根本原因是它缺乏共价键,这意味着它没有在中红外范围内吸收光的分子振动。这种光学透明性使得光谱仪能够测量样品的谱图而不会受到周围材料的干扰。
红外基质的基本特性
要理解为什么KBr是传统选择,有必要了解是什么使任何材料适合用于红外分析的样品。理想的材料不能干扰测量本身。
红外透明性原理
红外光谱通过测量分子内共价键对红外光的吸收来工作,这会导致它们振动(伸缩、弯曲等)。
KBr是一种离子盐(K⁺Br⁻)。它不含共价键。因此,它没有可以被中红外辐射激发的分子振动,使其在最有用的分析区域对光谱仪来说实际上是不可见的。
样品制备的物理适用性
KBr是一种相对柔软的结晶盐。当将其研磨成细粉并承受高压(数吨)时,晶体变形并融合在一起。
这个过程会形成一个薄的、半透明或透明的固体圆盘,通常称为KBr压片。与KBr一起研磨的样品被困在这个固体盐基基质中,非常适合分析。
一致的折射率
制备良好的KBr压片具有均匀的折射率,有助于最大限度地减少红外光的散射。这种散射的减少导致更平坦的基线和更清晰、更易于解释的谱图。

理解权衡和常见陷阱
虽然KBr是传统的标准,但它并非没有挑战。了解这些局限性对于生成高质量数据至关重要。
吸水问题
KBr最显著的缺点是它具有吸湿性,这意味着它很容易从大气中吸收水分。
水(H₂O)具有非常强、宽的红外吸收带,特别是O-H伸缩振动在3400 cm⁻¹附近。如果您的KBr没有保持完全干燥,这些水峰可能会掩盖样品谱图中的重要特征。
劳动密集型过程
制作高质量的KBr压片需要细心、技巧和时间。样品和KBr必须研磨成极细的粉末以减少光散射,均匀混合,并小心压制以形成清晰、无裂纹的压片。
潜在的样品相互作用
对于某些类型的样品,例如一些无机盐,在压力下存在与KBr基质发生离子交换反应的风险。这可能会改变样品并产生不准确的谱图。
KBr方法的现代替代方案
仪器设备的进步提供了强大的替代方案,绕过了与KBr压片相关的挑战。
衰减全反射(ATR)
ATR现在是固体和液体红外分析最常用的技术。它几乎不需要样品制备。
样品只需压在具有高折射率的晶体(通常是金刚石或硒化锌)上。红外光束在晶体内部反射,其一小部分能量穿透样品,生成谱图。这种方法快速、简便且无损。
石蜡油糊(Nujol Mull)
一种较旧的技术包括将固体样品与几滴矿物油(石蜡油)一起研磨,形成浓稠的糊状物。然后将这种糊状物涂抹在两块盐片之间(可以是KBr或NaCl制成)。
主要缺点是矿物油本身具有C-H吸收带,这些吸收带将始终出现在谱图中,可能会掩盖样品指纹区的一部分。
为您的分析做出正确选择
选择正确的样品制备技术完全取决于您的样品、您的设备和您的分析目标。
- 如果您的主要目标是为固体样品获得最高质量的经典透射光谱: KBr压片法,如果在干燥环境中正确执行,仍然是金标准。
- 如果您的主要目标是速度、易用性和最少的样品制备: 衰减全反射(ATR)几乎总是常规分析的卓越现代选择。
- 如果您的样品对压力敏感或可能与KBr反应: 考虑石蜡油糊技术或ATR作为更安全的替代方案。
理解您选择基质背后的原理,使您能够生成清晰、可靠且可解释的光谱数据。
总结表:
| 特性 | 为什么它对红外光谱很重要 |
|---|---|
| 红外透明性 | 无共价键 = 在4000–400 cm⁻¹范围内无干扰 |
| 吸湿性 | 吸收水分,需要仔细干燥以避免水峰 |
| 压片形成 | 在压力下为固体样品创建透明基质 |
| 折射率 | 最大限度地减少光散射,获得更清晰的基线 |
需要可靠的红外光谱解决方案? KINTEK专注于实验室设备和耗材,用于精确的样品制备和分析。无论您需要KBr压片、ATR附件,还是优化光谱结果的专家指导,我们的产品都能确保您实验室的准确性和效率。立即联系我们,讨论您的具体需求并提升您的红外能力!
图解指南
相关产品
- 实验室材料与分析金相试样镶嵌机
- 带加热板的自动加热液压压机,用于实验室热压 25T 30T 50T
- 单冲电动压片机 实验室粉末压片机 TDP压片机
- 手动高温加热液压压机带加热板用于实验室
- 小型工件生产用冷等静压机 400Mpa



















