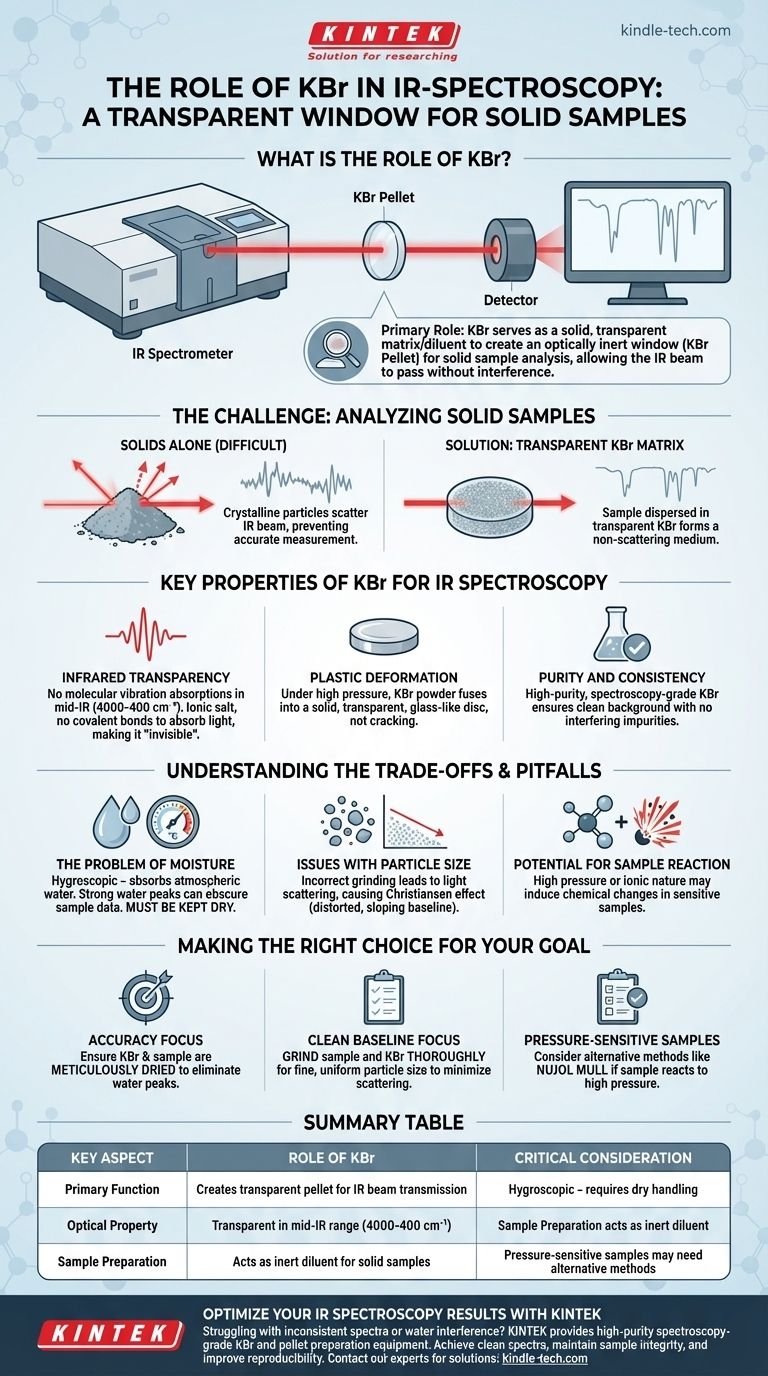
在红外(IR)光谱学中,溴化钾(KBr)用作固体基质或稀释剂,用于承载固体样品进行分析。其主要作用是创建一个小的、透明的窗口——称为KBr压片——让仪器的红外光束能够穿过。固体样品被精细研磨并与纯KBr粉末混合,然后在高压下压缩形成此压片。
KBr的核心功能是为固体样品提供一个光学惰性环境。由于KBr对红外辐射是透明的,它允许光谱仪测量样品本身的吸收光谱,而不会受到周围基质的干扰。
基本挑战:分析固体样品
红外光谱学依赖于测量样品的化学键如何吸收红外光。为了实现这一点,红外光束必须能够干净地穿过样品。
为什么固体难以分析
与可以放置在简单透明池中的液体或气体不同,固体粉末不能直接分析。结晶颗粒会散射红外光束,从而阻碍准确的吸收测量。
解决方案:透明基质
为了克服这个问题,固体样品被均匀地分散在一种不干扰测量的介质中。这种介质必须在红外区域透明,并在制备时形成固体、不散射的介质。这正是KBr所扮演的角色。

KBr在红外光谱学中的关键特性
KBr并非唯一可用的材料,但由于其物理和光学特性的理想组合,它是最常见的。
红外透明性
KBr最关键的特性是其在红外中区(4000-400 cm⁻¹)没有分子振动吸收。因为它是一种简单的离子盐,在该区域没有吸收红外光的共价键,使其对光谱仪来说实际上是不可见的,并为样品的光谱创建了一个清晰的窗口。
塑性变形
当承受高压(每平方英寸数吨的量级)时,KBr粉末具有独特的塑性流动特性。这意味着它会融合在一起形成一个坚固、透明、玻璃状的圆盘,而不是开裂或碎裂。
纯度和一致性
高纯度、光谱级KBr易于获得。这确保了不存在可能在光谱中引入不必要峰的杂质,从而提供了一个干净可靠的背景。
理解权衡与陷阱
虽然KBr压片技术功能强大,但并非没有挑战。正确的制备对于获得高质量光谱至关重要。
水分问题
KBr具有吸湿性,这意味着它很容易从大气中吸收水分。水具有非常强、宽的红外吸收带,很容易掩盖样品的重要峰。因此,KBr必须保持完全干燥,通常通过将其储存在干燥器或烘箱中。
粒度问题
如果样品研磨不够细或与KBr混合不均匀,其颗粒会散射红外光。这会导致基线扭曲、倾斜,并降低光谱质量,这个问题被称为克里斯蒂安森效应(Christiansen effect)。
样品反应的可能性
用于形成压片的极高压力有时会引起样品的化学反应或相变。此外,KBr的离子性质可以与某些类型的样品相互作用,改变其光谱。
根据您的目标做出正确选择
使用KBr时,正确的技术至关重要,以确保您的结果准确且可重现。
- 如果您的主要关注点是准确性: 确保您的KBr和样品在制备前都经过精心干燥,以消除干扰的水峰。
- 如果您的主要关注点是干净的基线: 将样品和KBr彻底研磨在一起,以获得细小、均匀的粒度,从而最大程度地减少光散射。
- 如果您怀疑您的样品对压力敏感: 考虑替代的固体采样方法,例如制备石蜡油糊(Nujol mull),它不需要高压。
最终,理解KBr作为惰性光学窗口的作用是掌握这种基本光谱技术的关键。
总结表:
| 关键方面 | KBr的作用 |
|---|---|
| 主要功能 | 创建透明压片以传输红外光束 |
| 光学特性 | 在中红外区域(4000-400 cm⁻¹)透明 |
| 样品制备 | 作为固体样品的惰性稀释剂 |
| 关键考虑因素 | 吸湿性 - 需要干燥处理 |
| 替代用途 | 压敏样品可能需要替代方法 |
使用KINTEK优化您的红外光谱结果
您是否正在为不一致的红外光谱或固体样品分析中的水干扰而苦恼?KINTEK专注于高纯度实验室设备和耗材,确保准确的光谱结果。
我们的光谱级KBr和压片制备设备帮助您:
- 获得具有最佳透明度的干净、无干扰光谱
- 通过适当的干燥和处理解决方案保持样品完整性
- 通过一致、高质量的材料提高重现性
无论您是分析药品、聚合物还是研究样品,KINTEK都能为您的实验室提供精确红外光谱所需的可靠实验室设备和耗材。
立即联系我们的专家,讨论您的具体应用需求,并发现我们的解决方案如何增强您的分析能力!
图解指南