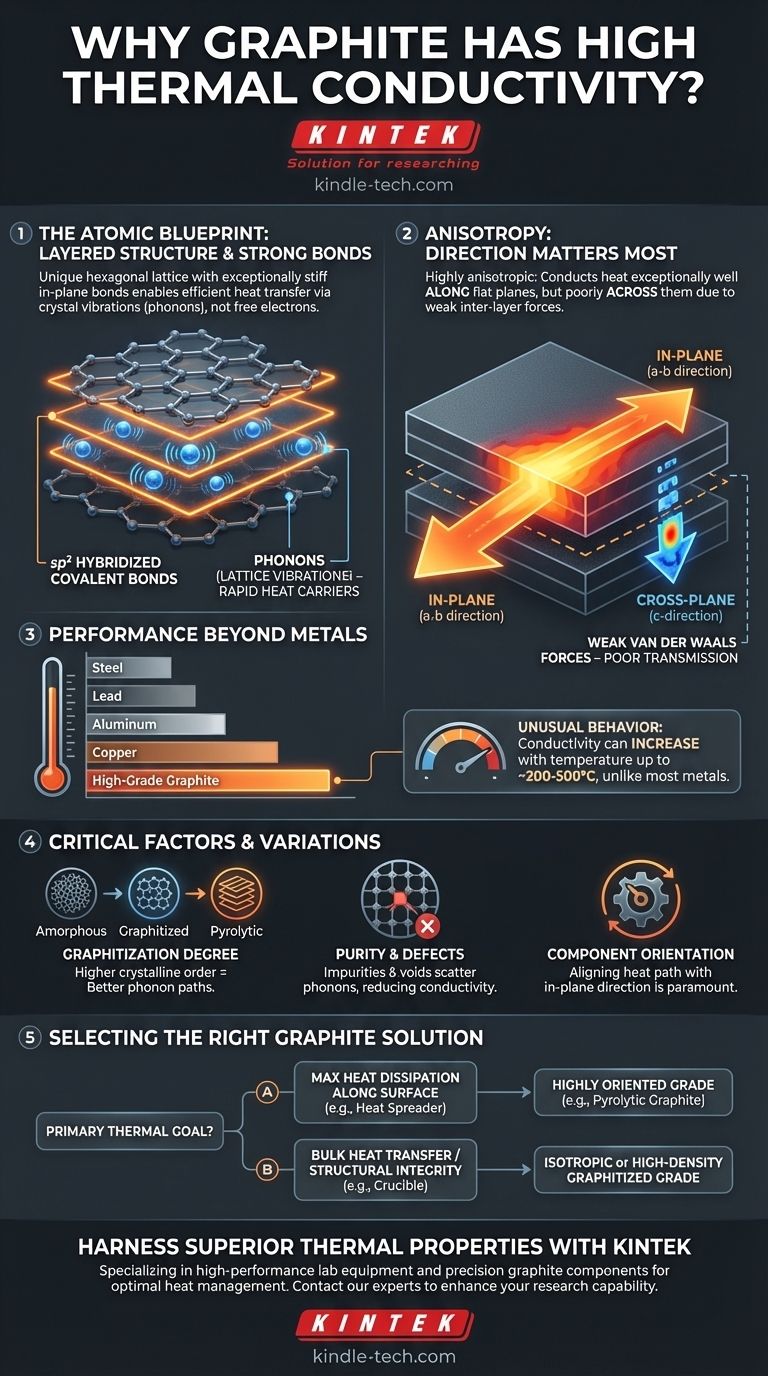
其核心在于,石墨的高导热性源于其独特的分层原子结构。这些层内的强共价键使得热能(以晶格振动的形式)能够以极快的速度和极小的阻力传播,就像声音穿过绷紧的鼓皮一样。
关键在于理解石墨的导热性并非均匀的。它是一种高度各向异性的材料,这意味着它沿着其平面导热性能极好,但垂直于平面导热性能很差。这种方向性是其在实际应用中最重要的因素。
导热的原子蓝图
石墨之所以能超越包括钢和铅在内的许多金属,不是因为像金属那样的自由电子,而是因为其晶格内物理振动的效率。
sp² 杂化键的作用
石墨层中的每个碳原子与六角形晶格中的另外三个碳原子键合。这些是sp² 杂化键——与石墨烯等其他碳同素异形体中发现的强键类型相同。这些键极其坚固,形成了一个坚硬的平面。
作为热载体的晶格振动(声子)
在像石墨这样的非金属固体中,热量主要通过声子(量化的振动能量包)来传递。想象一下敲击一个钟;你听到的声音就是能量以振动的形式在材料中传播。
当石墨晶格的一部分被加热时,其原子会更剧烈地振动。由于面内键非常牢固,结构非常有序,这些振动会以极小的能量损失有效地传递给相邻的原子。
各向异性:两个方向的故事
石墨性能的秘密在于其两个截然不同的结构特征:
- 面内(a-b 方向): 平坦的六角形层具有极高的导热性。热量沿着这些平面快速传播。
- 垂直于平面(c 方向): 这些层堆叠在一起,并通过非常弱的范德华力结合在一起。这些弱键在层间传递振动的能力很差,导致层间的导热性显著降低。
这种差异可能非常巨大,面内导热率有时比垂直于平面的导热率高出数百倍。
与其他材料的性能比较
石墨的导热性能常常是违反直觉的,特别是与我们通常认为导热性好的金属相比。
超越常见金属
如前所述,特定等级石墨的导热系数大于铁、钢和铅。高质量石墨的导热性甚至可以与铜和铝相媲美,尤其是在按重量计算时,使其成为轻量化热管理领域的更优选择。其导电性也很高,通常与其导热性能相关。
温度因素
与导热系数通常随温度升高而降低的金属不同,许多等级的石墨表现出不寻常的行为。它们的导热系数在达到一定点(通常在 200-500°C 左右)之前会随温度升高而增加,然后才开始下降。这使得石墨在金属效率降低的高温应用中特别有用。
理解权衡和变化
选择石墨并非一刀切的解决方案。其有效性完全取决于材料的等级及其在最终应用中的取向。
各向异性的关键影响
最常见的错误是未能考虑到石墨的方向性导热。如果一个部件的设计要求热量流过石墨层(c 方向)而不是沿着它们(a-b 方向),性能将远低于预期。正确的取向至关重要。
并非所有石墨都生而平等
“石墨”一词涵盖了各种材料。
- 无定形碳: 结构无序,导热性非常低。
- 石墨化碳: 在极高温度(超过 2500°C)下进行热处理的材料,以形成更有序的晶体结构。石墨化程度越高,导热性越高。
- 热解石墨: 一种高度有序的形式,具有极端的各向异性,提供了一些最高的面内导热率。
纯度和缺陷的作用
晶格中的杂质、空隙和缺陷会破坏声子传播的清晰路径。它们充当阻碍热流的“散射点”。因此,更纯净、更完美的晶体结构(如高等级合成石墨中所发现的)将始终具有卓越的导热性。
为您的应用做出正确的选择
选择正确的石墨等级和取向对于成功至关重要。您的决定应以您需要解决的主要热挑战为指导。
- 如果您的主要关注点是沿着表面的最大散热(例如,散热器): 使用高度取向的等级,如热解石墨,确保材料的平面与所需的热流路径对齐。
- 如果您的主要关注点是多个方向的大容量热传递(例如,坩埚): 具有所有方向上更均匀特性的各向同性石墨或金属浸渍复合材料等级可能是更好的选择。
- 如果您的主要关注点是具有良好热管理的耐高温结构完整性: 高纯度、高密度的石墨化等级将提供机械强度和导热性的平衡。
通过了解石墨的原子结构与其热性能之间的联系,您可以选择精确的材料来满足您的工程目标。
总结表:
| 特性 | 面内(a-b 方向) | 垂直于平面(c 方向) |
|---|---|---|
| 键类型 | 强 sp² 共价键 | 弱范德华力 |
| 导热系数 | 极高 | 显著较低 |
| 主要热载体 | 声子(晶格振动) | 声子(传递效率低) |
准备好在您的实验室中利用石墨卓越的热性能了吗?
在 KINTEK,我们专注于提供高性能的实验室设备和耗材,包括专为最佳热管理而设计的精密石墨部件。无论您需要散热器、坩埚还是定制的高温解决方案,我们的专业知识都能确保您获得正确的等级和取向,以实现最高效率。
让 KINTEK 成为您创新的合作伙伴。 立即联系我们的专家,讨论我们的石墨解决方案如何增强您实验室的能力并推动您的研究向前发展。
图解指南